Scientists have found an avenue that can simultaneously address the air/water-instability and structural-cum-electrochemical instability of Sodium–transition-metal–oxide-based cathode materials for Sodium-ion batteries and, accordingly, have developed new air/water-stable stable and high-performance cathode materials. The newly developed materials exhibit high electrochemical cyclic stability and stability upon exposure to air/water, thus, facilitating the development of systems that are expected to serve as cost-effective and sustainable energy storage systems for a range of applications, including consumer electronic devices, grid energy storage, storage of energy harvested from renewables and, eventually, electric vehicles.
As the importance of battery-driven electric vehicles increases due to climate and environmental concerns, development of a cost-effective, resource-friendly, safe, and sustainable alkali metal-ion battery system beyond the Li-ion system is essential. India has an abundance of Na-sources, which renders the upcoming Na-ion battery system extremely important in the Indian context. Like any alkali metal-ion battery cell, a Na-ion cell has cathode and anode active materials (supported on metallic current collector foils), which facilitate reversible insertion/removal of the charge carrier (viz., Na-ion) during charge/discharge of the cell. In such a cell, the cathode material initiates as the Na-reservoir, with the cell performances depending on the structural/electrochemical stability of the electrodes, Na-transport kinetics, and various resistances (which are dynamic in nature).
Despite the many advantages of Sodium-ion batteries, the electrochemical behaviour/performances of the ‘layered’ Na-TM-oxide-based cathode materials and their stability upon moisture exposure need substantial improvements for widespread development and usage of Na-ion battery systems for a variety of applications to be a reality. This is because such lack of stability renders handling/storage of Na-TM-oxides challenging and also negatively affects their electrochemical performance. Besides, the water instability mandates the usage of toxic-hazardous-expensive chemicals like N-Methyl-2-pyrrolidone (NMP) for electrode preparation, as opposed to the possible usage of water-based slurries.
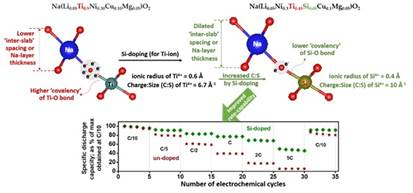
Prof Amartya Mukhopadhyay’s group at IIT Bombay, in their recent research, exploited materials science and electrochemical principles to reveal the dominant factors and controlling parameters that can help develop high-performance Na-ion batteries in order to address this challenge. In their research supported by the Science and Engineering Research Board (SERB), an attached institution of the Department of Science and Technology (DST) and DST’s Materials for Energy Storage scheme, they have evolved a universal design criterion, paving the way towards successful design and widespread development of environmentally stable and high-performance cathodes for the sustainable Na-ion battery system and beyond. The researchers have suggested a change in the alternate slab layered structure of the Na-TM-oxide structure of the Na-ion battery cathode by introducing “interslab” spacing by tuning the TM-O bond covalency in a paper published in the journal Chemical Communications.
The typical ‘layered’ Na-TM-oxide structure is built of alternate ‘slabs’ composed of NaO2 (O-Na-O) and TMO2 (O-TM-O), with the O-ions (which bear a net negative charge) being shared by the TM-ions and the Na-ions in their respective layers (the O-ion is common to both the cations). Here, while the TM-O bond is iono-covalent in nature, the Na-O is predominantly ionic. In such a ‘layered’ structure, tuning the degree of covalency of the TM-O bond by designing a suitable combination of cations in the TM-layer can tune the net/effective negative charge on the O-ion, which, in turn, can affect the electrostatic attraction between the Na- and O-ions and also the repulsions between the O-ions across the Na-layer.
